Blogpost
Students build rocket system
In contrast to my first entry’s futuristic outlook, I intend to return to the reality of now with the following words: “Where can I start building a spaceship?” For some very...
In contrast to my first entry’s futuristic outlook, I intend to return to the reality of now with the following words: “Where can I start building a spaceship?”
For some very lucky students at Purdue University of Lafayette, Illinois, the answer to that question is an excited “Right here, silly!” As part of NASA’s Project Morpheus, several graduate students have taken up the challenge to build a propulsion system, capable of landing a spacecraft on the Moon and then lifting the fuel-depleted lander for its voyage home.
You can read about their work in the press release, but what I’m really interested in is the science behind their propulsion system.
Liquid-propellant rockets
The basic design of their rocket is a liquid-propellant rocket, using liquid methane as fuel and liquid oxygen as an oxidizer. Now, I never had any formal education in liquid-propellant rockets (I sure wish I did though), so I don’t immediately know what that means. We’ll start our research with a bit of history. According to the Wikipedia article on liquid rockets, the concept was envisioned around the beginning of the twentieth century by a completely awesome Russian scientist Konstantin Tsiolkovsky in a book modestly titled “The Exploration of Cosmic Space by Means of Reaction Devices”. If you are a science history buff, NASA translated the book to English in 1975 and made it available on the internet.
This was the idea on Tsiolkovsky mind: “We shall imagine such a missile: metallic oblong chamber (of the form of least resistance), provided with light, oxygen, absorbers of carbon dioxide, miasmata and other animal secretions, meant not only for the preservation of different physical devices, but also for human beings, controlling the chamber (we shall discuss the question more widely according to possibility). The chamber has a larger reserve of substances, which on their mixing immediately form explosive mass. These substances, accurately and satisfactorily evenly exploding at the fixed place for the purpose, flow in the form of hot gases in the tubes, widening toward the end like the horn or the blowing musical instrument. These tubes are located along the small walls of the chamber, in the direction of its length. At one, narrow end of the tube the mixing of the explosive substances takes place: here the thickened and flaming gases are obtained. At the other, widened end of it, they, being vigorously rarefied and cooled, escape outside from the tube through the socket with enormous relative speed. It is understood, that such a missile, as a rocket, in known conditions, will be lifted to the altitude.”
He adds in an “Assembly required” fashion, that: “Automatic devices controlling the motion of the rocket (we shall often call our device thus) and the force of explosion for the plan contemplated earlier, are required.”
There is some strange content regarding animal secretions in there, the brilliant meaning of which surely got lost in translation, but the general idea of liquid-propellant rockets is there, unchanged since.
Tsiolkovsky recalled later in life, that he was inspired by another amazing human being, science fiction pioneer Jules Verne, who, in Tsiolkovsky’s words: “... directed my thought along certain channels, then came a desire, and after that, the work of the mind.” As a side note, Tsiolkovsky went deaf at age nine, lost his mother at age 13 and was left alone with a poor father. He nevertheless thought himself mathematics and got accepted to Moscow’s technical college, where his life as the pioneer of modern rocket theory began. But I digress.

Konstantin Tsiolkovsky was more of a theoretical kind of guy, and we have to fast forward 23 years, to March 16, 1926, when we are greeted by another pioneer of the rocket age, Robbert H. Goddard, and the first official liquid-propellant rocket flight. Lasting 2.5 seconds, it wasn’t the longest flight on record, but it proved that liquid rockets were possible.

On the image above, Goddard is seen standing next to the first liquid rocket “Nell”, where it isn’t immediately clear where the rocket ends and the rocket stand begins. Turns out that this particular rocket design is counter-intuitive, at least to the untrained eye, since modern rockets have spoiled us by having combustion nozzles at their bottom parts. Goddard’s rocket, on the other hand, has a combustion chamber and nozzle above the fuel tank (the bottom rocket-looking part), which is protected from the explosion by an asbestos cone. The whole rocket is thus much taller than Goddard himself, contrary to initial intuition.
Goddard improved his design in the following years and ultimately reached 2.7km in 1937 with his L-B type rocket. While that was the maximum performance Goddard had gotten out of his rockets, his work was very influential for many future liquid rocket designs. Most notable of these was, perhaps, the German WWII V-2 rocket, designed by Wernher von Braun.
How does it work?
The principle of a liquid rocket engines in use today is much the same as when it was envisioned by Tsiolkovsky in 1903. The rocket contains two tanks with two substances in liquid form, that act as the fuel and oxidizer. These two substances are actively pumped into the combustion chamber, where their mixture creates an explosion. The violent expansion of gases in the explosion is used to propel the rocket onwards. Simple as that! While indeed simple in principle, the high-temperature, high-velocity and other extreme conditions these rocket engines are usually operating in, their implementation is extremely complex.
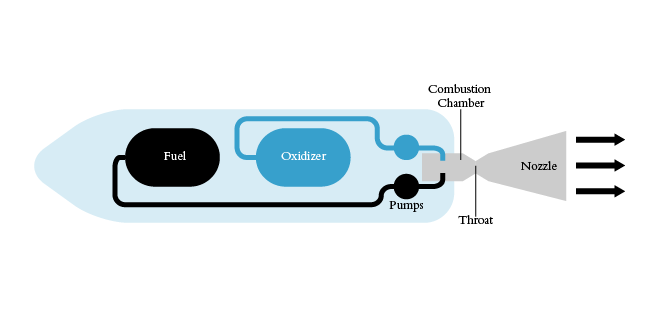
And now things are finally falling into place. Purdue University students are using liquid methane as fuel, and that’s a very important engineering decision. Methane has never been used to power a spacecraft, but it has several advantages over currently used fuels (e.g. liquid hydrogen): 1. It can be stored at a cozy -161°C (compared to hydrogen’s -252°C), tanks that carry it need less insulation and can be lighter. 2. Dense (denser than liquid hydrogen), which again computes to lighter tanks. 3. Methane is relatively safe for humans, no hazmat suit required to handle it. 4. Methane is abundant and could be manufactured on future worlds.
It does have at least one major shortcoming though: it has to be ignited in order to burn. Liquid hydrogen, for example, will ignite spontaneously upon contact with the oxidizer in the combustion chamber. This ignite-to-burn property of methane complicates the rocket engine and poses one of the biggest challenges for it.
On a side note, there is a very impressive video of a liquid methane rocket test available, showing an XCOR Aerospace rocket.
Can I build a liquid rocket engine myself?
Well that is entirely up to you. There is a very good guide on how to design, build and test small liquid-fuel rocket engines available on-line, aptly titled “How to Design, Build and Test Small Liquid-Fueled Rocket Engines”. You’ll probably have to start with something a lot cheaper and easier to handle than liquid oxygen or methane, something like gaseous oxygen and gasoline. But that already sounds like lots of fun.
Maybe you’ll be the one to build the next generation liquid rocket, that will take us to Mars and beyond. I can’t see why not.